Sandra Malavaud, Head of Infection Control Departments, University-hospital Center of Toulouse-rangueil, and Cancer University Institute-oncopôle, France
Pathogenic microorganisms, including those which are multi-resistant to anti-infectious products, can persist, often over long periods, in a poorly or badly cleaned hospital environment. Cleaning always entails detergents, and often disinfection. Disinfection is only optimal if the detergence has also been optimal. The main techniques entail use of often complex products and combined detergent-disinfectant products, of bleach and water vapor. The selection process should take into account the effectiveness of products and technical tools, their cost, the feasibility of their implementation, and their innocuousness for cleaning personnel, patients and the environment. Staff training is the cornerstone of effective realization of satisfactory safety conditions.
The role of the environment in cross-transmission and infectious disease outbreaks
The role of the environment in the spread of infectious diseases, especially enteric diseases, has been known for a long tim e in community outbreaks. In hospital structures, the role of surfaces and objects contaminated by bacteria (1, 2), such as methicillin-resistant Staphylococcus aureus (MRSA), has been amply demonstrated (3, 4), particularly in the occurrence of epidemic outbreaks (5). The control of cross-transmission of MRSA has especially benefited from improved hand hygiene with the development of hydro-alcohol hand rubbing and from sanitizing the hospital environment, the latter deserving more attention than has been the case up to now (6).
But improving hand hygiene has not had the same impact on bacteria of intestinal origin, both because some of these bacteria are not destroyed by alcohol (e.g., C. difficile), and because the environmental contamination is much greater, on account of the enormous inoculum which the intestinal reservoir represents (109 to 1011 CFU / gram of feces, including 106 to 108 enterobacteria (7, 8), the multiplicity of bacterial species, as well as their ability to acquire resistances which are numerous as well as based on many various mechanisms. This is the case of bacteria, particularly Enterobacteriaceae, which secrete extended-spectrum beta-lactamase (ESBL) (9) and/or carbapenemases, or glycopeptide-resistant Enterococcus faecium (ERG), whose presence in the environment is well established (10). As they are part of the intestinal microbiota, dissemination of these species is favoured by a lack of personal hygiene and hand hygiene (real “dirty hands” diseases), but also by frequent clinical situations in our patients, such as diarrhea and faecal incontinence. It is the same for intestinal pathogenic viruses, such as Norovirus (11, 12). The spore-forming anaerobic bacteria (Clostridium difficile), due to their low sensitivity to conventional cleaning processes, are a real problem, the source of many epidemics and large amounts of antibiotics prescriptions, in particular glycopeptides (13, 14). The faecal peril is back. Finally, other pathogenic Gram-negative bacilli, increasingly resistant to antibiotics, can also colonize both dry environments (Acinetobacter baumannii), (15, 16) and wet environments (Pseudomonas aeruginosa), (17, 18).
Multi-resistant bacteria have become a major challenge for public health as they may, whenever provoking illness, lead to a therapeutic impasse for affected patients (already estimated to be causing hundreds of thousands of extra deaths per year, globally), so much so that WHO estimates that this problem is likely to question the most spectacular achievements of medicine of the past half century.
Many publications establish their presence in the environment of carrying patients and their persistence in the environment, in vegetative or spore form, and for significant although variable periods, depending on the microorganism, can range from days to even (and often) several months (19, 20). A stay in the same room as a patient carrying MRSA is a factor for MRSA acquisition (21). The increased risk of acquisition of a microbial strain by a patient located in a room immediately after a carrier-patient with this strain has also been demonstrated (21), more than double for those Gram-negative (ESBL, Acinetobacter, Pseudomonas) and almost double for those Gram-positive (MRSA, glycopeptide-resistant enterococci (22). The quality of cleaning, at the time of the patient departure is a key element in controlling the risk of cross-transmission, and the spread of antimicrobial resistance.
Increased attention is needed on the cleaning and disinfection step process
The problems caused by these bacteria are of concern not only for the cleaning maintenance of surfaces near patients, or that of mattresses (23), but also the management of excreta, including cleaning and disinfection of toilets, showers and sinks (17). With the aim of containing the spread of multi-resistant bacteria, increasing attention should be paid to these issues (24).
We can only disinfect well that which is clean. When a surface has a microbial population the acts of cleaning and disinfection aim at reducing, by successive steps, the number of bacteria initially present at each step level; moreover, most disinfecting agents’ effectiveness is reduced in the presence of soils, whether organic and/or mineral. The cleaning step is a key element to optimize, whenever necessary, the disinfection step. Other factors may be involved, such as temperature and hardness of the water used for the possible dilution of products. It is thus essential to perform the following successive steps:
- Humide cleaning with a detergent product so as to remove soils by chemical and mechanical action and reduce the number of microorganisms, including those which adhere to surfaces as biofilms. The role of biofilm on dry surfaces and the loss of susceptibility to biocides is a booming topic (25). To date, standardized, pertinent and appropriate tests are lacking, leading to an obvious difficulty in the choice of products, often with cost as the sole criterion.
- Descaling: when it comes to faucets or fixtures, this step is important because of the negative interaction of these deposits with biocides, and their role in biofilm formation.
- Rinsing: an indispensable addition to the removal of soils and of some of the microorganisms, it helps to prevent the risk of incompatibility between products.
- Disinfection: its role significantly reduces the microbial populations still present after the cleaning step.
The choice of a product or procedure with physical and/or chemical biocidal properties
Numerous procedures, with physical and/or chemical biocides have been proposed. Disinfectant products are thus generally tested according to standardized protocols, in the laboratory, usually with bacteria, viruses, yeasts and fungi, spores, supposedly representative of pathogenic microorganisms and in conditions also deemed representative (NF EN 14885, October 2015). The biocidal activity in the medical field is tested on microbial suspensions (Standard Phase I) in the presence of interfering substances (Standard Phase II-1) or on microorganisms deposited on a surface (Standard Phase II-2). The product can claim a “-cide” activity when it is able to ensure a population reduction at a level defined by the microorganism and the relevant standard (5 log for bacteria). The actual comparison of their respective effectiveness is, however, complicated by the lack of standardization of the tests in real conditions of use, on the ground, in real life situations.
Today, the choice of a product or procedure can thus only be based on laboratory tests, far from reflecting the actual conditions of use. In addition to its purchase price and staff time associated with the use, it should also take into account the toxicity to users and the cost of individual protection measures and equipment, as well as the environmental impact to air, and where appropriate, to the effluents.
Many “all in one” products, “detergents-disinfectants” or even “detergents-descalers-disinfectants”, are on the market, arousing a strong craze among hospital managers due to their alleged savings in labour time; however, they do not entail a reduction stepwise as described above, and the method of application does not provide for rinsing at any time. It is conceivable that actual efficiency in real usage is less than that observed in the laboratory. Their composition is often complex, they are not devoid of toxic risk for users (26, 27), their prices remain high and they are not present in low-income countries, where sodium hypochlorite is still the main disinfectant used.
The case of bleach
The market for sodium hypochlorite being essentially a domestic market, and in use for a very long time, manufacturers have not, to our knowledge, felt the need to make the tests described above. It remains, however, the most effective disinfectant (10, 28, 29, 30, 31), and the disinfectant recommended with respect to the most epidemic-prone microorganisms and/or those less sensitive to chemical processes, such as bacterial spores (C. difficile) (10, 29) and filamentous spores (Aspergillus spp), naked virus (Norovirus) (30) or hemorrhagic fever viruses, such as ebola, which is an enveloped virus, yet with a high level of contamination, including faecal contamination (31). Sodium hypochlorite is also extremely efficient for cleaning surfaces in countries with high levels of hepatitis and HIV, notably as antimicrobial resistance includes the worrisome increase in HIV resistance to antiretrovirals. The rehabilitation of hypochlorite is a common sense necessity in high-income countries where its reputation suffers from episodic consequences of misuse by users (release of chlorine gas by mixing the hypochlorite with other products, poor conditions in solution preparations). Solutions such as wipes have sometimes been proposed to improve the satisfaction of staff and patients (33), but the duration of activity has yet to be demonstrated. The training of staff responsible for cleaning, in both techniques and products is an imperative, a guarantee of efficiency and lack of accidents.
Water vapor, a promising technique?
Already in full swing for its effectiveness in the detergency step, water vapor has so far lacked standardized tests to also demonstrate its action as a disinfectant. A specific working group has been created within the French Agency for Standardization (AFNOR), and the standard is being validated, allowing for demonstration of a spectrum of activity in the recommended conditions of use (suitable applicators, set speed).
Thus, preliminary tests were carried out which show that water vapor is bactericidal, yeasticidal, fungicidal, and mycobactericidal on mandatory strains, as well as on multi-antibiotic-resistant strains isolated in the course of clinical practice in our hospital (pan-resistant Pseudomonas aeruginosa, multi-resistant Acinetobacter baumanii, Extended Spectrum beta-lactamase (ESBL)-producing Klebsiella pneumoniae, carbapenemase carrying Enterobacter cloacae, and additional strains of interest (Geotrichum candidum, Aspergillus flavus) and several viruses, including naked viruses (Murine Norovirus, Adenovirus Type 5 and Coronavirus) (Sanivap, biotech-Germande, Fonderephar Nov. 2015, currently in publication).
This process is completely safe for the environment and for staff (the risk of burns being mastered once the staff have been trained in the use of the steam generator); it has been shown to be time saving compared to the techniques used in our establishment, storage is facilitated by the lack of storage products, its only drawback is the need for an electrical connection which can be a limiting factor in some countries or under certain conditions.
The procedure using steam with punctually added hydrogen peroxide is now also available, bringing a significant expansion in its spectrum effectiveness: efficiency is demonstrated for spores of Bacillus cereus and C. difficile. The coupling of procedures allows for a sporicidal efficiency with low doses of hydrogen peroxide, in comparison with the doses used to disinfect surfaces through air systems. Thus, repeated measurements in environmental room settings in the course of utilisation, have found rates way below the reference value limit, which is reassuring regarding the lack of toxicity risk to personnel duly equipped with a surgical mask and safety glasses. However, the addition of hydrogen peroxide to water vapor seems to us to be warranted under certain specific indications, for example, at the time of discharge from hospitalization for a patient with an episode of C. difficile diarrhea.
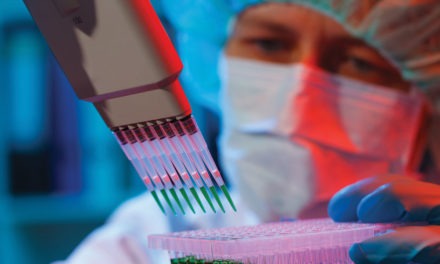
Table I summarizes the characteristics of these three methods.
Other procedures are available, with or without human presence: disinfection of surfaces through the air with hydrogen peroxide mist (34–37), ultraviolet light (38–40), incorporation of biocide or metals (copper, silver, titanium dioxide) having antimicrobial properties, in materials (door handles, paint and wall coverings, etc.) (41) electrolyzed water (42). However, their indications, feasibility and the conditions for their implementation, their effectiveness, short-term and long-term, and the potential risks need to be better defined.
The qualifications of the personnel in charge of cleaning
The last – but not the least – element to consider is the qualifications of the personnel in charge of cleaning. This activity is wrongly not often taken into consideration, the task is being handed over to under-skilled or totally unskilled, poorly paid and unmotivated staff, either internal or outsourced subcontractors. This leaves the cleaning personnel without the keys to understanding the importance of their work for the institution; an importance not reflected in the organization of their training. Yet as powerful as the chosen bio cleaning procedure may be in a health institution, it will be nothing without trained and motivated staff.
The stabilization of cleaning crews, their professional qualifications and their training, both initial and continuing, are crucial to the effectiveness of bio cleaning techniques (10, 23). It is necessary to define a basic corpus of knowledge allowing these personnel to understand the risk of acquiring potentially pathogenic microorganisms, for themselves and for the patients, to understand the effectiveness of cleaning and disinfection of surfaces and of the environment in reducing the risks, on the one hand, and of the possible adverse health effects of multiple products in accordance with numerous active ingredients, more or less complex formulations, presentations such as mace, liquids, concentrated powders, methods of preparation and application, ventilation and size of the premises, on the other hand (43) and the means to protect themselves.
Joint work between hygienists and occupational physicians should define not only the content but also the most appropriate training methods, cognitive inputs and practical implementation, either in real situation settings, or by simulation: relevant choices in products, possible dilutions, application methods (wipe, spray, etc.), use of special equipment (steam generators, for example), protective clothing (and donning and doffing as needed) for users, proper cleaning techniques, respecting the principle of gradual steps (the cleanest to the dirtiest) management of cleaning and disinfection products supplies and stocks, maintenance and storage of cleaning equipment, and waste disposal. The development of simple protocols and simple pictorial technical specifications, will reinforce the knowledge acquired in their practical implementation in everyday life. Finally, a regular assessment of practical and visual results with feedback to the interested parties, should be a strong point in an institutional quality approach.
Conclusion
The hospital environment as a reservoir and source of cross-transmission of microorganisms is a phenomenon that cannot be ignored. Along with hand hygiene, the importance of which has been recognized as a central element in the fight against healthcare-associated infections and has led to the implementation of specific prevention programmes, it is imperative today to see that cleaning and routine maintenance of the hospital environment should become a major, cost-effective component in the control of healthcare-associated infections and rising antimicrobial resistance. It should focus on safe techniques (efficacy, safety) and the less expensive ones. Research must be intensified in this field, the actions of specific training to become a priority in healthcare institutions. Finally, through clear recommendations and national prevention programmes adapted to local constraints, governments should encourage and strongly support improved cleaning and disinfection practices in health facilities.
Biography
TO download article click here
References
1. Russotto V, Cortegiani A, Raineri SM, Giarratano A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. Journal of Intensive Care. 2015;3:54
2. Otter JA, Yezli S, Salked JA, French GL. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control. 2013;41(5suppl.)S6-11
3. Boyce J.M. Environmental contamination makes an important contribution to hospital infection. J. Hospit Infect. 2007;65,S2:50-4
4. Sexton T, Clarke P, O’Neil E, Dillane T, Humphreys H. Environmental reservoirs of methicillin-resistant Staphylococcus aureus in isolation rooms: correlation with patient isolates and implication form hospital hygiene. J. Hospit Infect. 2006;62:187-94
5. Otter JA, Klein JL, Watts TL, Kearns AM, French GL. Identification and control of an outbreak of ciprofloxacin-susceptible EMRSA-15 on a neonatal unit. J. Hospit Infect. 2007;67:232-239
6. Dancer SJ. Importance of the environment methicillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. J. Intensive Care. 2015;3:54doi:10.1186/s40560-015-0120-5
7. Collignon A, Butel M-J. Etablissement et composition de la flore microbienne intestinale, in Flore microbienne intestinale Physiopathologie et pathologies digestives, 2004, John Libbey Euritext, coordonné par Rambaud JC, Buts JP, Corthier G, Flourié B. pg.247
8. Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis. 2004;39:219-26
9. Guet-Revillet H, Le Monnier A, Breton N, Descamps Ph, Lecuyer H, Alaabouche I, Constance Bureau BS, Nassif X, Zahar JR. Environmental contamination with extended-spectrum B-lactamases : is there any difference between escherichia coli and Klebsiella spp? Am J Infect Control. 2012;40:845-8
10. Eckstein BC, Adams DA, Eckstein EC, Rao A, Sethi AK, Yadavali GK, Donskey CJ. Reduction of Clostridium difficile and vancomycin-resistant Enterococcus contamination of environmental surfaces after an intervention to improve cleaning methods. BMC Infectious Diseases. 2007;7:61
11. Barker J, Vipond IB, Bloomfield SF. Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via surfaces, J. Hospit Infect. 2004;58:42-49
12. Weber DJ, Rutala WA, Miller MB, Huslage K, Sickert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficle and Acinetobacter species. J. Hospit Infect. 2009;73;4:378-85
13. Barbut F. How to eradicate Clostridium difficile from the environment. J Hospit Infect. 2015; 89:287-295
14. Khanafer N, Voirin N, Barbut F, Kuipjer E, Vanhems P. Hospital management of Clostridium difficile infection: a review of the literature. J. Hospit Infect. 2015;90:91-101
15. Zenati K, Touati A, Bakour S, Sahli F, Rolain JM. Characterization of NDM-1- and OXA-23-producing Acinetobacter baumannii isolates from inanimate surfaces in a hospital environment in Algeria. J. Hospit Infect. 2016;92:19-26
16. Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358:1271-81
17. Manian FA, Griesnauer S, Senkel D. Impact of terminal cleaning and disinfection on isolation of Acinetobacter baumannii complex from inanimate surfaces of hospital rooms by quantitative and qualitative methods. Am J Infect Control. 2013;41384-5
18. Ambrogi V, Cavalie L, Mantion B, Ghiglia M-J, Cointault O, Dubois D, Prere MF, Levitzki, Kamar N, Malavaud S. Transmission of metallo-B-Lactamase-producing Pseudomonas aeruginosa in a nephrology-transplant unit with potential link to the environment. J. Hospit Infect. 2016;92:27-29
19. Wendel AF, Kolbe-Busch S, Ressina S, Schulze-Röbekke R, Kindgen-Milles D, Lorenz C, Pfeffer K, MacKenzie CR. Detection and termination of an extended low-frequency hospital outbreak of GIM-1-producing Pseudomonas aeruginosa ST111 in Germany. Am J Infect Control. 2015;43:635-9
20. Kramer A, SchwebkeI, Kampf G. How long do nosocomial pathogenes persist on inanimate surfaces? A systematic review. BMC Infectious Diseases 2006;6:130
21. Zarpellon MN, Gales AC, Sasaki AL, Selhorst GJ, Menegucci TC, Cardoso CL, Garcia LB, Tognim MCB. Survival of vancomycin-intermediate Staphylococcus aureus on hospital surfaces. J. Hospit Infect. 2015;90:347-50
22. Moore C, Dhaliwal J, Tong A, Eden S, Wigston C, Willey B, McGeer A. Risk factors for methicillin-resistant Staphylococcus aureus (MRSA) acquisition in roommate contacts of patients colonized or infected with MRAS in an acute-care hospital. Infect Control Hosp Epidemiol. 2008;29;7:600-6
23. Mitchell BG, Dancer SJ, Anderson M, Dehn E. Risk of organism acquisition from prior room occupants: a systematic review and meta-analysis. J. Hospit Infect. 2015:91:211-217
24. Hopman J, Hakizimana B, Meintjes WAJ, Nillessen M, de Both E, Voss A, Mehtar S. Manual cleaning of hospital mattresses: an observational study comparing high- and low-resource settings. J. Hospit Infect. 2016;92:14-18
25. Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. Lancet Infect Dis. 2008; 8,2:101-13
26. Otter JA, Vickery K, Walker JT, deLancey Pulcini E, Stoodley P, Goldenberg SD, Salked JAG, Chewins J, Yezli S, Edgeworth JD. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. J. Hospit Infect. 2015;89:16-27
27. Gonzalez M, Jégu J, Kopferschmitt MC, Donnay C, Hedelin G, Matzinger F, Velten M, Guilloux L, Cantineau A, de Blay F. Asthma among workers in healthcare settings: role of disinfection quaternary ammonium compounds. Clinical &Experimental Allergy. 44:393-406
28. Vizcaya D, Mirabelli MC, Gimeno D, Anto JM, Delclos G, Rivera M, Orriols R, Arjona L, Burgos F, Zock JP. Cleaning products and short-term respiratory effects among female cleaners with asthma. Occup Environ Med. 2015;0:1-7
29. Grabsch EA, Mahony AA, Cameron DR, Martin RD, Helan M, Davey P, Petty M, Xie S, Grayson ML. Significant reduction in vancomycin-resistant enterococcus colonization and bacteraemia after introduction of a bleach-based cleaning-disinfection programme. J. Hosp Infect. 2012;82;4:234-42
30. Hacek DM, Ogle AM, Fischer A, Robicsek A, Peterson LR. Significant impact of terminal room cleaning with bleach on reducing nosocomial Clostridium difficile. Am J Infect Control. 2010;38;5:350-3
31. Chiu S, Skura B, Petric M, McIntire L, Gamage B, Isaac-renton J. Efficacy of common disinfectant/cleaning agents in inactivating murine norovirus and feline calicivirus as surrogate viruses for human norovirus. Am J Infect Control. 2015;43:1208-12
32. Judson S, Prescott J, Munster V. Understanding Ebola virus transmission. Viruses. 2015;7:511-521;doi:10.3390/v7020511
33. Rutala WA, Weber DJ. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin Microbiol Reviews. 1997;10;4:597-610
34. Arhonalt KC, McManus J, Orenstein R, Faller R, Link M. Patient and environmental service employee satisfaction of using germicidal bleach wipes for patient room cleaning. Journal for Healthcare Quality. 2012;35;6:30-36
35. French GL, Otter JA, Shannon KP, Adams NMT, Watling D, Parks MJ. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J Hospit Infect. 2004;57:31-37
36. Bates CJ, Pearse R. Use of hydrogen peroxide vapour for environmental control during a Serratia outbreak in a neonatal intensive care unit. J Hospit Infect. 2005;doi:10.1016/j.jhin.2005.05.003
37. Dryden M, Parnaby R, Dailly S, Lewis T, Davis-Blues K, Otter JA, Kerans AM. Hydrogen peroxide vapour decontamination in the control of a polyclonal methicillin-resistant Staphylococcus aureus outbreak on a surgical ward. J Hospit Infect. 2007; doi:10.1016/j.jhin.2007.11.003
38. Otter JA, Cummins M, Ahmad F, van Tonder C, Drabu YJ. Assessing the biological efficacy and rate of recontamination following hydrogen peroxide vapour decontamination. J. Hospit Infect. 2007;67:182-188
39. Petersson LP, Albrecht UV, Sedlacek L, Gemein S, Gebel J, Vonberg RP. Portable UV light as an alternative for decontamination. Am J Infect Control. 2014;42:1334-6
40. Jinadatha C, Villamaria FC, Ganachari-Mallappa N, Brown D, Liao IC, Stock E, Copeland LA, Zeber JE. Can pulsed xenon ultraviolet light systems disinfect aerobic bacteria in the absence of manual disinfection? Am J Infect Control. 2015;43:415-7
41. Jinadatha C, Villamaria FC, Restrepo MI, Ganachari-Mallappa N, Liao IC, Stock E, Copeland LA, Zeber JE. Is the pulsed xenon ultraviolet light no-touch disinfection system effective on methicillin-resistant Staphylococcus aureus in the absence of manual cleaning? Am J Infect Control. 2015;43
42. Muller MP, MacDougall C, Lim M, and the Ontario Agency for health protection and promotion (Public Health Ontario) Provincial Infectious Diseases Advisory Committee on Infection Prevention and control (PIDAC-IPC). Antimicrobial surfaces to prevent health-care associated infections: a systematic review. J. Hospit Infect. 2016;92:7-13
43. Dancer SJ. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014;27:665-690
44. Quinn MM, Henneberger PK, and the members of the National Institute for Occupational Safety and Health (NIOSH), National Occupational Research Agenda (NORA). Cleaning and Disinfecting in Healthcare Working Group Cleaning and disinfecting environmental surfaces in health care: toward an integrated framework for infection and occupational illness prevention. Am J Infect Control. 2015;43:424-34